Softener
- Home
- Softener
Sources of Hardness Minerals in Drinking Water
Water is a good solvent and picks up impurities easily. Pure water — tasteless, colorless, and odorless — is often called the universal solvent. When water is combined with carbon dioxide to form very weak carbonic acid, an even better solvent results. As water moves through soil and rock, it dissolves very small amounts of minerals and holds them in solution. Calcium and magnesium dissolved in water are the two most common minerals that make water “hard.” The degree of hardness becomes greater as the calcium and magnesium content increases and is related to the concentration of multivalent cations dissolved in the water..
Every household and every factory uses water, and none of it is pure.
One class of impurity that is of special interest is “hardness”. This refers to the presence of dissolved ions, mainly of calcium Ca 2+ and magnesium Mg 2+ which are acquired through contact with rocks and sediments in the environment. The positive electrical charges of these ions are balanced by the presence of anions (negative ions), of which bicarbonate HCO 3 – and carbonate CO 3 2– are most important. These ions have their origins in limestone sediments and also from carbon dioxide which is present in all waters exposed to the atmosphere and especially in groundwaters.
Indications of Hard Water
Hard water interferes with almost every cleaning task from laundering and dishwashing to bathing and personal grooming. Clothes laundered in hard water may look dingy and feel harsh and scratchy. Dishes and glasses may be spotted when dry. Hard water may cause a film on glass shower doors, shower walls, bathtubs, sinks, faucets, etc. Hair washed in hard water may feel sticky and look dull. Water flow may be reduced by deposits in pipes.
Dealing with hard water problems in the home can be a nuisance. The amount of hardness minerals in water affects the amount of soap and detergent necessary for cleaning. Soap used in hard water combines with the minerals to form a sticky soap curd. Some synthetic detergents are less effective in hard water because the active ingredient is partially inactivated by hardness, even though it stays dissolved. Bathing with soap in hard water leaves a film of sticky soap curd on the skin. The film may prevent removal of soil and bacteria. Soap curd interferes with the return of skin to its normal, slightly acid condition, and may lead to irritation. Soap curd on hair may make it dull, lifeless and difficult to manage.
When doing laundry in hard water, soap curds lodge in fabric during washing to make fabric stiff and rough. Incomplete soil removal from laundry causes graying of white fabric and the loss of brightness in colors. A sour odor can develop in clothes. Continuous laundering in hard water can shorten the life of clothes. In addition, soap curds can deposit on dishes, bathtubs and showers, and all water fixtures.
Hard water also contributes to inefficient and costly operation of water-using appliances. Heated hard water forms a scale of calcium and magnesium minerals that can contribute to the inefficient operation or failure of water-using appliances. Pipes can become clogged with scale that reduces water flow and ultimately requires pipe replacement.
Potential Health Effects
Hard water is not a health hazard. In fact, the National Research Council (National Academy of Sciences) states that hard drinking water generally contributes a small amount toward total calcium and magnesium human dietary needs. They further state that in some instances, where dissolved calcium and magnesium are very high, water could be a major contributor of calcium and magnesium to the diet.
Researchers have studied water hardness and cardiovascular disease mortality. Such studies have been “epidemiological studies,” which are statistical relationship studies.
While some studies suggest a correlation between hard water and lower cardiovascular disease mortality, other studies do not suggest a correlation. The National Research Council states that results at this time are inconclusive and recommends that further studies should be conducted.
Testing
If you are on a municipal water system, the water supplier can tell you the hardness level of the water they deliver. If you have a private water supply, you can have the water tested for hardness. Most water testing laboratories offer hardness tests for a fee, including the Environmental Quality Center. Also many companies that sell water treatment equipment offer hardness tests. When using these water tests, be certain you understand the nature of the test, the water condition being measured, and the significance of the test results. An approximate estimate of water hardness can be obtained without the aid of outside testing facilities. Water hardness testing kits are available for purchase through water testing supply companies. If more accurate measurements are needed, contact a testing laboratory.
Interpreting Test Results
The hardness of your water will be reported in grains per gallon, milligrams per liter (mg/l) or parts per million (ppm). One grain of hardness equals 17.1 mg/l or ppm of hardness.
The Environmental Protection Agency (EPA) establishes standards for drinking water which fall into two categories — Primary Standards and Secondary Standards.
Primary Standards are based on health considerations and Secondary Standards are based on taste, odor, color, corrosivity, foaming, and staining properties of water. There is no Primary or Secondary standard for water hardness. Water hardness is classified by the U.S. Department of Interior and the Water Quality Association as follows:
| Classification | mg/l or ppm | grains/gal |
| Soft | 0 – 17.1 | 0 – 1 |
| Slightly hard | 17.1 – 60 | 1 – 3.5 |
| Hard | 120 – 180 | 7.0 – 10.5 |
| Very Hard | 180 & over | 10.5 & over |
NOTE:
Other organizations may use slightly different classifications.
Options
There are two ways to help control water hardness: use a packaged water softener or use a mechanical water softening unit.
Packaged water softeners are chemicals that help control water hardness. They fall into two categories: precipitating and non-precipitating.
Precipitating water softeners
include washing soda and borax. These products form an insoluble precipitate with calcium and magnesium ions. The mineral ions then cannot interfere with cleaning efficiency, but the precipitate makes water cloudy and can build up on surfaces. Precipitating water softeners increase alkalinity of the cleaning solution and this may damage skin and other materials being cleaned.
Non-precipitating water softeners
use complex phosphates to sequester calcium and magnesium ions. There is no precipitate to form deposits and alkalinity is not increased. If used in enough quantity, non-precipitating water softeners will help dissolve soap curd for a period of time.
Mechanical water softening units can be permanently installed into the plumbing system to continuously remove calcium and magnesium. Water softeners operate on the ion exchange process. In this process, water passes through a media bed, usually sulfonated polystyrene beads. The beads are supersaturated with sodium. The ion exchange process takes place as hard water passes through the softening material. The hardness minerals attach themselves to the resin beads while sodium on the resin beads is released simultaneously into the water. When the resin becomes saturated with calcium and magnesium, it must be recharged. The recharging is done by passing a salt (brine) solution through the resin. The sodium replaces the calcium and magnesium which are discharged in the waste water. Hard water treated with an ion exchange water softener has sodium added. According to the Water Quality Association (WQA), the ion exchange softening process adds sodium at the rate of about 8 mg/liter for each grain of hardness removed per gallon of water.
For example
if the water has a hardness of 10 grains per gallon, it will contain about 80 mg/liter of sodium after being softened in an ion exchange water softener if all hardness minerals are removed.
Because of the sodium content of softened water, some individuals may be advised by their physician, not to install water softeners, to soften only hot water or to bypass the water softener with a cold water line to provide unsoftened water for drinking and cooking; usually to a separate faucet at the kitchen sink.
Softened water is not recommended for watering plants, lawns, and gardens due to its sodium content.
Although not commonly used, potassium chloride can be used to create the salt brine. In that case potassium rather than sodium is exchanged with calcium and magnesium.
Before selecting a mechanical water softener, test water for hardness and iron content. When selecting a water softener, the regeneration control system, the hardness removal capacity and the iron limitations are three important elements to consider.
There are three common regeneration control systems. These include a time-clock control (you program the clock to regenerate on a fixed schedule); water meter control (regenerates after a fixed amount of water has passed through the softener); and hardness sensor control (sensor detects hardness of the water leaving the unit, and signals softener when regeneration is needed).
Hardness removal capacity, between regenerations, will vary with units. Softeners with small capacities must regenerate more often. Your daily softening need depends on the amount of water used daily in your household and the hardness of your water. To determine your daily hardness removal need, multiply daily household water use (measured in gallons) by the hardness of the water (measured in grains per gallon).
Example:
400 gallons used per day X 15 grains per gallon hardness = 6,000 grains of hardness must be removed daily.
Iron removal limitations will vary with water softener units. If the iron level in your water exceeds the maximum iron removal capacity recommended by the manufacturer of the unit you are considering, iron may foul the softener, eventually causing it to become plugged.
How Softeners Work
Below is a diagram of our Softener. It shows how our softener, and everyone else’s, works. A water softener is an ion exchanger.
Hard water—water with a high calcium/magnesium content—enters the softener through the “In” port indicated by the green arrow. It passes through the control valve and into the tank, where it goes from top to bottom through a specially prepared resin that “softens” it.
The resin consists of specially manufactured beads that have been saturated with sodium ions. “Softening” occurs as the hardness minerals in the water attach themselves to the resin and are “exchanged” for sodium.
The softened water then enters the long center tube, called a riser, via the strainer basket in the bottom of the tank and passes upward through the riser. The water exits the softener via the control valve (blue arrow) and is sent to the home.
When the resin becomes saturated by hardness minerals, the softener automatically goes into regeneration. (The regeneration process is initiated by a timer or a meter, depending on the type of softener you purchase.) By this process the hardness minerals are washed down the drain (via a drain tube not shown in the diagram), and the resin bed is rinsed, resettled, and recharged with sodium. It is now again ready to soften your water.
The regeneration process is accomplished by passing very salty water from the brine tank through the resin.
The brine tank must remain filled with softener salt at all times so that it can regenerate the softening resin again and again
Water Softeners Make Water Work Better
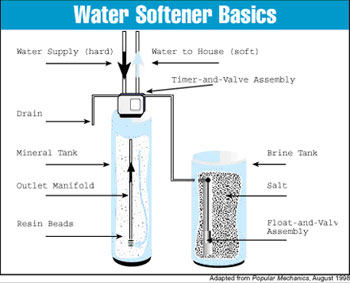
Water softeners combat this nuisance by eliminating the minerals that cause hard water. The most common kind of water softener is a mechanical appliance plumbed directly into the home’s water supply intake. (See figure 1) The water softener exchanges calcium and magnesium with sodium in a process called ion exchange.
The water softening system consists of a mineral tank and a brine tank. The water supply pipe is connected to the mineral tank so that water coming into the house must pass through the tank before it can be used.
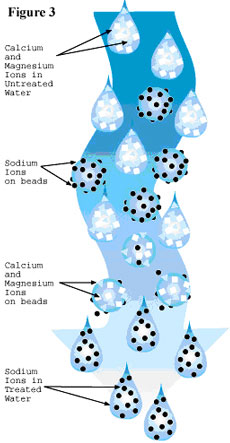
The mineral tank holds small beads (also known as resin) that carry a negative electrical charge. The positively charged calcium and magnesium (called ions) are attracted to the negatively charged beads. This attraction makes the minerals stick to the beads as the hard water passes through the mineral tank. (See figure 3.)
Eventually the surfaces of the beads in the mineral tank become coated with the calcium and magnesium minerals. To clean the beads, a strong sodium (salt) solution held in the brine tank is flushed through the mineral tank. Sodium ions also have a positive electrical charge, just not quite as strong as that of calcium and magnesium. This large volume of sodium ions overpowers the calcium and magnesium ions and drives them off of the beads and into the solution. The sodium solution carrying the minerals is then drained out of the unit. Some sodium ions remain in the tank attached to the surfaces of the beads
Conventional water softening
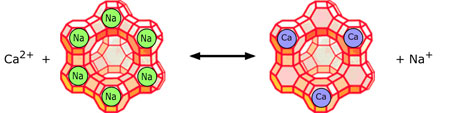
Most conventional water-softening devices depend on a process known as ion-exchange in which “hardness” ions trade places with sodium and chloride ions that are loosely bound to an ion-exchange resin or a zeolite (many zeolite minerals occur in nature, but specialized ones are often made artificially.)
The illustration depicts a negatively-charged zeolite to which [positive] sodium ions are attached. Calcium or magnesium ions in the water displace sodium ions, which are released into the water. In a similar way, positively-charged zeolites bind negatively-charged chloride ions (Cl –), which get displaced by bicarbonate ions in the water. As the zeolites become converted to their Ca 2+ and HCO 3 – forms they gradually lose their effectiveness and must be regenerated. This is accomplished by passing a concentrated brine solution though them, causing the above reaction to be reversed. Herein lies one of the drawbacks of this process: most of the salt employed in the regeneration process gets flushed out of the system and and is usually released into the soil or drainage system— something that can have damaging consequences to the environment, especially in arid regions. For this reason, many jurisdications prohibit such release, and require users to dispose of the spent brine at an approved site or to use a commercial service company.
The Softening Process
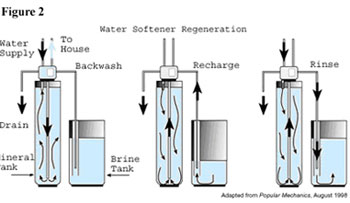
The normal water softening cycle operates like this: Hard water enters the mineral tank. Inside the tank, the calcium and magnesium ions carried in the water attach themselves to the beads. The surfaces of the beads eventually hold their limit of calcium and magnesium and can’t remove any more from the water. At this point the water softener must be “regenerated”. (See figure 2.) The three-step regeneration cycle can be scheduled according to a timer or by a flow detection meter.
The first step, called the backwash phase, reverses the water’s flow and flushes any accumulated dirt particles out of the tank and down the drain. Next, in the regeneration or recharge phase, the sodium rich brine solution flows from the brine tank into and through the mineral tank. The brine washes the calcium and magnesium off the beads. In the final phase, the mineral tank is flushed of the excess brine, which now also holds the calcium and magnesium, and the solution is disposed of down the drain.
Sodium ions from the previous regeneration cycle cling to the beads. Now when the hard water flows into the mineral tank, the calcium and magnesium ions change places with the sodium ions on the resin. The displaced sodium ions remain dissolved in the water.